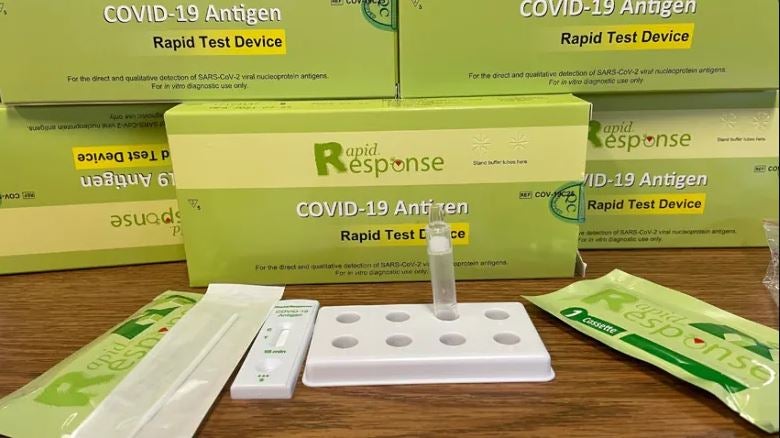
3 Test Kits of BTNX Covid-19 Antigen Rapid Test Device, Nasal/Nasopharyngeal Secreations-Individually packed test devices, 25 tests/KIT. Total= 75 Tests MEDLEE
$ 299,99 $ 120,00
Shipping immediately. Pickup in downtown Toronto is available – same day. All new orders will be shipped within 2-10 business days.
Product Code: COV-19C25
Sample: Nasal/Nasopharyngeal secretions
Format: Cassette
Quantity: 25 Tests/Kit
Expired 2023/2024
Time to result: 15 minutes
Storage Condition: 2-30°C/36-86°F
Test Principle: Immunochromatographic Assay
Contents:
- Individually packed test devices
- Extraction Buffer
- Extraction tube
- Nozzle with filter
- Tube stand
- Individually packed swabs
- Package Insert
Product Description:
The Rapid Response™ COVID-19 Antigen Rapid Test Device is an in vitro immunochromatographic assay for the direct and qualitative detection of SARS-CoV-2 viral nucleoprotein antigens from nasal and nasopharyngeal secretions from individuals suspected of COVID-19 within 6 days of symptom onset and from individuals without symptoms or other epidemiological reasons to suspect COVID-19 infection, when tested twice over two (or three) days with at least 24 hours (and no more than 36 hours) between tests. This test is authorized for use at the Point of Care i.e., in patient care setting.
Results are for the identification of SARS-CoV-2 viral nucleoprotein antigen. Antigens are generally detectable in nasopharyngeal and nasal secretions during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Laboratories are required to report all positive results to the appropriate public health authorities.
Negative results should be treated as presumptive, and do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of a patient’s recent exposures, history, and the presence of clinical signs and symptoms consistent with COVID-19, and confirmed with a molecular assay, if necessary, for patient management.
The Rapid Response™ COVID-19 Antigen Rapid Test Device is intended for use by trained laboratory personnel or health care professionals.
Pursuant to section 5 of the Interim Order Respecting the Importation and Sale of Medical Devices for Use in Relation to COVID-19, made by the Minister of Health on March 18, 2020, the Rapid Response™?COVID-19 Antigen Rapid Test Device is now authorized for sale or importation in Canada. Interim Authorization Number: 321669
*All Covid-19 Antigen Rapid Test Device products may be substituted for another brand of a similar quality if we’re out of stock due to the shortage. All products are final sale. No cancellations. No returns or exchanges.
Please view/download:
Public Health Ontario-Testing Yourself for COVID-19
Health Canada Authorization Reference Number : 321669
COVID-19 Antigen Rapid Test Device Product Insert
Procedure Card COVID-19 Antigen Rapid Test Device
BTNX – Point of Care
*About COVID-19 Antigen Rapid Test Device
The COVID-19 Antigen Rapid Test Device detects the presence of SARS-CoV-2 viral nucleoprotein antigens in nasal and nasopharyngeal secretions. In just 15 minutes, individuals can determine a positive or negative result. If positive, individuals should adhere to their local public health guidelines and verify results with a PCR test.
Please view/download:
Public Health Ontario-Testing Yourself for COVID-19
Prompt Shipping and professional packaging
We offer a broad range of shipping options thanks to our long-standing relationships with UPS, FedEx and DHL. Our warehouse personnel will pack every item to our exacting specifications. Your goods will go through an extensive inspection and will be adequately secured before being shipped. We ship to thousands of customers each day across different countries. The fact that we are committed to becoming the biggest online retailer in the World is obvious. Both Europe as well as the USA have distribution and warehouse centers.
Orders that contain more than one item are assigned processing times in accordance with the item.
Prior to shipment, all purchased products will be thoroughly inspected. Most orders are now shipped within 48 hours. The delivery estimate is 3 to 7 days.
Returns
We don't manage the stock at our factory and warehouse. So the actual stock may fluctuate at any moment. It's possible that the stocks could be depleted after your order has been placed.
Our policy lasts for 30 days. We cannot return or exchange your purchase if it has been 30 days from the date of purchase.
To be considered eligible for return it must be in its original packaging, unopened and in the condition you received it. The item must be in the original packaging.
Related products
Medical Products
Medical Products
Medical Products
KF94 Protective Mask, 4 layers protection, 3D structure design, Box of 30pcs MEDLEE
Medical Products
Medical Products
Medical Products
Medical Products
Sharp Collector/Container BD 22.7L Nestable Funnel Entry Top MEDLEE
Medical Products
Medical Products
Medical Products
Medical Products
Medical Products
Hands-free Automatic hand sanitizer dispenser with stand MEDLEE
Medical Products
Medical Products
Medical Products
Medical Products
Medical Products
Medical Products
Medical Products